In the microscopic world of cells, autophagy is a diligent “scavenger,” constantly maintaining a clean and stable intracellular environment. Spermidine is the core engine that powers this “scavenger”‘s efficient work. Through unique molecular mechanisms, it activates the autophagy pathway and safeguards cellular health.
(I) Molecular Activation Mechanism of the Autophagy Pathway
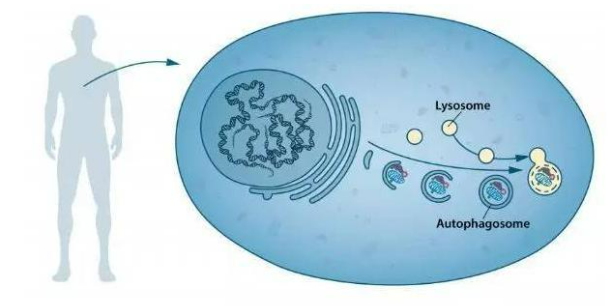
Spermidine’s activation of the autophagy pathway is a delicate and complex molecular regulatory process. Like a precise conductor, it orchestrates the melody of autophagy activation by regulating the expression of a series of autophagy-related genes. Among these, genes such as ATG5/ATG7 show significant upregulation in response to spermidine. These genes act as key components of autophagosome assembly, their increased expression providing the necessary building blocks for autophagosome formation.
For example, the LC3 protein undergoes a remarkable transformation from LC3-I to LC3-II under the influence of spermidine. LC3-I originally exists in a soluble form in cells. During spermidine-induced autophagy, it binds to phosphatidylethanolamine and converts to the membrane-bound form LC3-II. This conversion acts as a specialized “coat” for the LC3 protein, enabling it to participate in the elongation and maturation of the autophagosome membrane. Ultimately, the autophagosome successfully encapsulates damaged mitochondria, abnormal protein aggregates, and other cellular “waste,” paving the way for subsequent clearance.
The 2016 Nobel Prize in Physiology and Medicine’s in-depth research on the mechanisms of autophagy further confirmed the importance of spermidine as a natural autophagy activator. Studies have shown a strong positive correlation between intracellular spermidine concentration and autophagy efficiency. Elevated intracellular spermidine concentrations significantly increase the number of autophagosomes generated and enhance autophagic activity, leading to more efficient clearance of harmful substances from the cell. This discovery provides strong scientific evidence for the role of spermidine in regulating autophagy.
(II) Metabolic Waste Removal and Cellular Homeostasis
During the life cycle of a cell, various metabolic waste products and damaged cellular structures are inevitably produced. If these “waste products” are not promptly removed, they will accumulate within the cell, impairing normal cell function and even leading to cell aging and death. The cellular autophagy mechanism activated by spermidine acts like an efficient waste disposal system for the cell, effectively removing these metabolic waste products and maintaining cellular homeostasis.
Studies in yeast, nematode, and mammalian cell models clearly demonstrate the remarkable performance of spermidine in metabolic waste removal. When spermidine was administered to these cell models, the number of cells positive for senescence-associated β-galactosidase (SA-β-gal) was significantly reduced, by as much as 30%-40%. SA-β-gal-positive cells are a key hallmark of senescent cells, and their reduction indicates that spermidine can effectively delay cellular aging.
Spermidine also significantly reduces the accumulation of lipofuscin in cells. Lipofuscin is a pigment granule composed of oxidized proteins and lipids that gradually accumulates within cells as they age. This accumulation not only occupies intracellular space but also causes oxidative stress, further damaging the cells. Spermidine promotes autophagy, accelerating the clearance of lipofuscin and mitigating its harmful effects on cells.
Furthermore, spermidine plays a key role in maintaining the integrity of organelle function. Damaged organelles, such as mitochondria and the endoplasmic reticulum, are promptly cleared or repaired through spermidine-induced autophagy. This provides an optimal environment for various metabolic reactions within the cell, ensuring the normal functioning of cellular processes such as biosynthesis and energy conversion. Much like a factory, efficient production is only possible if the equipment remains functioning properly. The proper functioning of organelles within cells maintains normal physiological functions and provides the metabolic space and material foundation necessary for cell repair and regeneration.
 Delaying the Aging Process: The Secret to Longevity from Model Organisms to Humans
Delaying the Aging Process: The Secret to Longevity from Model Organisms to Humans
Aging is a complex yet inevitable physiological process, like the chisel of time, leaving its mark on every aspect of life. From cellular senescence and apoptosis to the decline of tissue and organ function, the impact of aging is pervasive. However, the emergence of spermidine offers new hope for delaying the aging process. Numerous studies have shown that spermidine acts at multiple levels, delaying the aging process by activating autophagy, regulating epigenetics, and protecting telomeres, providing strong support for organisms’ path to longevity.
(I) Cross-species Lifespan-Extension Effects
Spermidine’s lifespan-extending effects have been widely demonstrated in multiple species, from simple model organisms to mammals. It is like a magical key, unlocking the door to longevity.
In experiments with fruit flies, when spermidine was added to their drinking water, these tiny flies exhibited astonishing changes, extending their lifespan by 15%-20%. As a commonly used model organism, fruit flies have a short lifespan and rapid reproduction rate, allowing scientists to observe the effects of spermidine on lifespan in a relatively short period of time. This result initially reveals the potential of spermidine in delaying aging.
Spermidine also demonstrated its potent effects when applied to middle-aged mice. Middle-aged mice are equivalent to 50-60 years old in humans, experiencing a period of accelerated aging. When these mice were supplemented with spermidine, their median lifespan was extended by 12%. This significant change demonstrates that even in middle-aged organisms, where the aging process has already accelerated, spermidine can still effectively delay aging and extend lifespan.
Spermidine’s lifespan-extending effects are not accidental; they are driven by profound molecular mechanisms. Research has found that these effects rely on the activation of the autophagy pathway and the improvement of mitochondrial function. The autophagy pathway acts as a “garbage removal system” within the cell, clearing out harmful substances such as damaged organelles and abnormal protein aggregates, maintaining a stable intracellular environment. By activating the autophagy pathway, spermidine enhances the cell’s self-repair and cleanup capabilities, thereby delaying cellular aging.
Mitochondria are the cell’s “energy factories,” and their proper function directly impacts the cell’s energy supply and metabolic levels. Spermidine improves mitochondrial function, increasing energy production efficiency and reducing cellular damage caused by oxidative stress. It’s like upgrading a factory’s equipment to enable more efficient operation. This not only provides cells with ample energy but also helps maintain normal physiological functions and slow the aging process.
Mechanistically, spermidine inhibits mTOR kinase activity, initiating a remarkable journey that affects the fate of cells. mTOR kinase is a key regulator of cell growth and metabolism. When its activity is inhibited, cells receive a unique signal and begin adjusting their metabolic patterns. This activates the expression of the Sirtuins family of “longevity genes.” Sirtuins act as “longevity guardians” within cells, regulating numerous crucial processes, including metabolism, stress response, and DNA repair. Under the influence of spermidine, the expression of Sirtuins increases, actively working to reset the cellular aging clock, slowing the aging process and laying the foundation for longevity.
(II) Epigenetics and Telomere Protection
In the microscopic world of cells, epigenetics and telomeres act as two guardians of cellular youth, and spermidine is a powerful ally. By regulating epigenetic modifications and protecting telomeres, it plays a key role in delaying cellular aging.
Epigenetic modification refers to a mechanism that regulates gene expression without altering the DNA sequence. DNA methylation is a key mechanism of epigenetic regulation. With aging, DNA methylation patterns change. Changes in methylation levels in some aging-related genes, such as p16INK4a, can lead to abnormal gene expression, accelerating cellular aging. The emergence of spermidine has brought a new twist to this process. Research has found that spermidine can regulate DNA methylation, restoring the normal expression of aging-related genes like p16INK4a. Like a precise “gene regulator,” spermidine, by adjusting the methylation status of genes, inhibits the overexpression of aging-related genes, thereby slowing the rate of cellular aging. Telomeres are specialized structures at the ends of chromosomes, acting like caps. They play a crucial role in maintaining chromosome stability and integrity. As cells divide, telomeres gradually shorten. When telomere shortening reaches a certain level, cells enter a state of senescence or apoptosis. Therefore, telomere length is considered a key marker of cellular aging. Spermidine has demonstrated remarkable telomere protection. In experiments with human fibroblast cultures, spermidine treatment of the cells produced surprising changes. The rate of telomere attrition decreased by 25%, indicating a significant slowing of telomere shortening. Furthermore, the number of cell passages increased by 30%, indicating enhanced cell proliferation and a delayed aging process. These results provide direct and compelling evidence for spermidine’s anti-aging effects, highlighting its crucial role in protecting telomeres and delaying cellular aging.
Cardiovascular Protection: Multidimensional Regulation of Vascular Rejuvenation
Cardiovascular disease is one of the leading global threats to human health, with high morbidity and mortality rates. From a pathophysiological perspective, the development and progression of cardiovascular disease involves a complex process involving endothelial dysfunction, atherosclerosis, and thrombosis. Spermidine, a naturally occurring polyamine, has recently demonstrated multidimensional regulatory effects in cardiovascular protection, offering new hope for the prevention and treatment of cardiovascular disease.
(I) Endothelial Cell Repair and Function Maintenance
Endothelial cells act like a protective membrane lining the vascular lining. They not only serve as a physical barrier between blood and the vessel wall but also secrete a variety of bioactive substances, playing a vital role in maintaining normal vascular function. When endothelial cells are damaged, it’s like a breach in a wall, triggering a chain reaction that impairs vasodilation and increases the risk of cardiovascular disease.
Spermidine plays a key role in promoting endothelial cell repair and maintaining its function. In in vitro cell experiments, researchers surprisingly found that spermidine significantly promoted endothelial cell proliferation. Specifically, spermidine-treated cells showed a 40% increase in the number of Ki67-positive cells. Ki67 is a marker closely associated with cell proliferation. An increase in Ki67-positive cells indicates that more cells have entered a proliferative state, providing a sufficient source of cells for repairing damaged vascular endothelial cells.
Spermidine also upregulates the expression of nitric oxide synthase (eNOS). Nitric oxide synthase acts like a “factory” for nitric oxide; its increased expression means that more nitric oxide can be produced. Nitric oxide, as a key vasodilator, has a potent vasodilatory effect. It rapidly diffuses into vascular smooth muscle cells, activates guanylate cyclase, and increases intracellular cGMP levels, causing vascular smooth muscle relaxation, reducing peripheral vascular resistance and improving vasodilation. This process is like “loosening” tight blood vessels, allowing blood to flow more smoothly.
Numerous clinical and epidemiological studies have also provided strong evidence for the protective effects of spermidine on cardiovascular health. Studies have shown that insufficient spermidine intake is significantly associated with a 35% increased risk of coronary heart disease and a 28% increased risk of stroke. This means that when spermidine levels are low, the cardiovascular system loses a protective shield, making it more vulnerable to disease. Appropriate spermidine supplementation can potentially reduce the risk of these cardiovascular diseases and safeguard the health of our heart and blood vessels.
(II) Anti-atherosclerosis and Lipid Metabolism Regulation
Atherosclerosis is the primary pathological basis of cardiovascular disease, and its development is closely linked to inflammation and lipid metabolism disorders. During this process, monocytes adhere to vascular endothelial cells and subsequently differentiate into macrophages. Macrophages ingest large amounts of oxidized low-density lipoprotein (ox-LDL) and gradually transform into foam cells. These foam cells accumulate, ultimately forming atherosclerotic plaques.
Spermidine effectively inhibits the development and progression of atherosclerosis by inhibiting the NF-κB inflammatory pathway. NF-κB is a key inflammatory regulator. When activated, it triggers a series of inflammatory responses and promotes the expression of monocyte adhesion molecules (such as VCAM-1). Spermidine acts like a brake, inhibiting NF-κB activity and thereby reducing the expression of monocyte adhesion molecules. This makes it difficult for monocytes to adhere to vascular endothelial cells, preventing the formation of foam cells and slowing the progression of atherosclerosis.
Spermidine also plays a key role in regulating lipid metabolism. It promotes reverse cholesterol transport mediated by hepatic ABCA1. ABCA1 acts as a transporter, transporting excess cholesterol out of the cell where it binds to apolipoprotein A-I to form high-density lipoprotein (HDL). HDL then transports the cholesterol to the liver for metabolism and excretion. In mouse studies, spermidine supplementation reduced arterial plaque size by 22% and decreased serum ox-LDL levels by 18%. This result shows that spermidine reduces the deposition of lipids in blood vessel walls by promoting reverse cholesterol transport, effectively inhibiting the development of atherosclerosis and restoring blood vessels to normal state.
Immunomodulation: A Two-Way Controller of Inflammatory Balance
In the human immune system, inflammation is a double-edged sword. Appropriate inflammation is a crucial defense mechanism against pathogen invasion, but excessive or persistent inflammation can damage the body and lead to various diseases. Spermidine plays a key role in immune regulation. Like a precise two-way controller, it skillfully modulates the inflammatory response according to the body’s immune status, maintaining immune balance and safeguarding the body’s health.
(I) Dynamic Balance of Pro-inflammatory and Anti-inflammatory Signaling
Spermidine’s modulation of pro-inflammatory and anti-inflammatory signaling is like a delicate symphony, with each note working in harmony to maintain the dynamic balance of the inflammatory response. In a lipopolysaccharide (LPS)-induced inflammation model, spermidine demonstrated potent anti-inflammatory properties. It inhibited the release of pro-inflammatory cytokines such as TNF-α and IL-6 by as much as 40%-50%. TNF-α and IL-6 are like the vanguards of the inflammatory response. Their excessive release can trigger a strong inflammatory response and lead to tissue damage. The presence of spermidine acts like a rein for these vanguards, effectively inhibiting their activity and thus reducing the intensity of the inflammatory response.
Spermidine can also enhance the secretion of anti-inflammatory factors such as IL-10. IL-10 is a key anti-inflammatory cytokine that inhibits inflammatory cell activation and reduces the release of inflammatory mediators, thereby exerting its anti-inflammatory effects. By enhancing IL-10 secretion, spermidine further strengthens the body’s anti-inflammatory capacity, thereby better controlling the inflammatory response.
In immunocompromised models, spermidine has an immune-boosting effect. It can promote T cell proliferation and optimize the CD4+/CD8+ ratio. T cells are key players in the immune system. CD4+ T cells primarily participate in immune regulation and helper functions, while CD8+ T cells are cytotoxic and can directly kill pathogen-infected cells or tumor cells. Spermidine enhances the immune system’s cellular immunity by promoting T cell proliferation and optimizing its proportion, thereby improving the body’s resistance.
Spermidine also boosts the phagocytic activity of macrophages, increasing the phagocytic index by 30%. Macrophages are the “scavengers” of the immune system, engulfing and clearing pathogens, senescent cells, and other foreign matter. By enhancing macrophage phagocytic activity, spermidine enables them to more effectively eliminate harmful substances from the body, thereby maintaining the body’s health. This bidirectional regulatory effect on immune cells under different immune states makes spermidine indispensable in maintaining immune balance.
(II) Curbing Age-Associated Inflammation
With aging, the body inevitably develops age-associated inflammation, a chronic “inflammatory storm” that silently damages various tissues and organs. The senescent cell secretory phenotype (SASP) is a key hallmark of age-associated inflammation. These senescent cells secrete large amounts of inflammatory factors, such as IL-8 and MCP-1, which trigger inflammatory responses in surrounding tissues, leading to an increase in systemic inflammatory burden. Spermidine acts like a fire extinguisher in this “inflammatory storm,” curbing age-related inflammation through a unique mechanism. Spermidine has the ability to eliminate senescent cells (senolysis). The accumulation of senescent cells in the body is a major cause of age-related inflammation. Spermidine can identify and eliminate these senescent cells, reducing their impact on surrounding tissues and thereby reducing the severity of the inflammatory response.
Spermidine also inhibits the release of SASP factors. By modulating intracellular signaling pathways, it reduces the secretion of inflammatory factors such as IL-8 and MCP-1, thereby curbing the development and progression of age-related inflammation at the source. In a mouse model, supplementation with spermidine reduced age-related chronic inflammation scores by 35%, a significant change that fully demonstrates spermidine’s effectiveness in curbing age-related inflammation. By reducing the systemic inflammatory burden in elderly individuals, spermidine helps maintain immune balance, slow the aging process, and improve their quality of life.
Neuroprotection: Multi-Target Defense of the Central Nervous System
The brain, as the “headquarters” of the human body, is crucial for its health. However, with aging and the influence of various external factors, the brain faces numerous challenges, including neurodegeneration and oxidative stress. Spermidine, with its unique multi-target defense mechanism, provides comprehensive protection for brain health and has become a research hotspot in the field of neuroprotection.
(I) Anti-Neurodegeneration
Neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, pose a serious threat to human health and quality of life. The development and progression of these diseases are often accompanied by abnormal protein aggregation and nerve cell damage. For example, Alzheimer’s disease is characterized by the deposition of Aβ amyloid protein and the hyperphosphorylation of tau protein. These pathological changes lead to nerve cell death and impaired synaptic function, ultimately causing cognitive impairment and memory loss.
In studies using Alzheimer’s disease models in mice, spermidine demonstrated significant anti-neurodegenerative properties. It was able to reduce Aβ amyloid protein deposition by up to 25%. This effect acts like a powerful boost to the brain’s “garbage disposal system,” promptly clearing out these abnormal protein deposits and reducing their toxic effects on nerve cells.
Spermidine also inhibits tau hyperphosphorylation, effectively pressing a pause button on the abnormal “modification” of tau, maintaining its normal function. Under normal conditions, tau promotes the assembly and stability of microtubules, maintaining the structure and function of nerve cells. However, when tau becomes hyperphosphorylated, it dissociates from microtubules, destabilizing them and impairing their normal function. By inhibiting tau hyperphosphorylation, spermidine protects microtubule stability, ensuring the normal function of nerve cells.
Spermidine’s effects have also been impressive in behavioral experiments. Mice treated with spermidine showed a 20% reduction in latency in the Morris water maze test. The Morris water maze test is a commonly used method for assessing spatial memory in animals. A shortened latency means that mice are able to find the platform hidden in the water more quickly, indicating that spermidine effectively improves spatial memory in mice.
Mechanistically, spermidine’s anti-neurodegenerative effects are closely related to autophagy-mediated protein clearance and maintenance of synaptic plasticity. Autophagy is an intracellular self-degradation and recycling mechanism that removes damaged organelles, misfolded proteins, and pathogens, maintaining a stable intracellular environment. By activating autophagy, spermidine promotes the clearance of Aβ amyloid and hyperphosphorylated tau, alleviating their toxic effects on neurons.
Spermidine also upregulates PSD-95 protein expression by 15%. PSD-95 is an important postsynaptic density protein that plays a key role in synaptic plasticity. Synaptic plasticity refers to the ability of synaptic structure and function to change in response to neuronal activity and is the neurobiological basis of learning and memory. Spermidine enhances synaptic stability and function by upregulating PSD-95 protein expression, promoting signaling between neurons and thereby improving cognitive function.
(II) Oxidative Stress and Blood-Brain Barrier Protection
Oxidative stress is a significant issue in the normal physiological activities of the brain. When the brain is exposed to stimuli such as ischemia-reperfusion injury and inflammation, it produces large amounts of reactive oxygen species (ROS), such as superoxide anions and hydroxyl radicals. These ROS are like a double-edged sword. Under normal circumstances, they participate in physiological processes such as cellular signaling and immune defense. However, when ROS production exceeds the cell’s antioxidant defense capacity, oxidative stress results, damaging intracellular biomolecules such as lipids, proteins, and DNA, leading to apoptosis and death of neurons.
Spermidine plays an important role in combating oxidative stress. It can elevate glutathione (GSH) levels, a key intracellular antioxidant that reacts with ROS, converting them into harmless substances, thereby reducing ROS damage to cells. Spermidine also inhibits the production of ROS in neuronal mitochondria. Mitochondria are the “energy factories” of cells and a major source of ROS. By inhibiting mitochondrial ROS production, spermidine mitigates the threat of oxidative stress to neurons at the source.
The blood-brain barrier (BBB), composed of brain microvascular endothelial cells, basement membranes, and astrocytes, is a crucial line of defense for the brain. It prevents harmful substances from entering the brain and maintains a stable internal environment. Damage to the BBB is like a breach in a wall, allowing inflammatory cells, pathogens, and toxins to enter the brain, triggering neuroinflammation and neuronal damage.
Spermidine enhances the expression of tight junction proteins (such as ZO-1) in brain microvascular endothelial cells. Tight junction proteins act as the “glue” that connects brain microvascular endothelial cells. Increased expression of these proteins strengthens the connections between endothelial cells, thereby maintaining the integrity of the BBB. In a model of ischemia-reperfusion, spermidine’s protective effect was particularly pronounced, reducing the rate of neuronal apoptosis by 40%. This suggests that spermidine protects the blood-brain barrier, reduces the damage to nerve cells caused by harmful substances, effectively reduces the apoptosis rate of neurons, and provides strong support for brain health.
Regulation of Reproductive Health: From Gametogenesis to Maintenance of Fertility Potential
Reproductive health is a crucial cornerstone of human reproduction and individual health. It encompasses the normal development of the reproductive system, the production and maturation of gametes, the conception process, and the maintenance of health throughout the reproductive cycle. In this complex and delicate physiological process, spermidine plays an indispensable role. Like a silent guardian, it meticulously regulates reproductive health at multiple levels, safeguarding the birth and continuation of life.
(I) Protection of Male Germ Cells
In the male reproductive system, sperm quality and function are directly related to fertility. High concentrations of spermidine in semen act like a sturdy “protective shield” for sperm, playing a crucial role in protecting their health and function.
Spermidine’s antioxidant effect is one of its key mechanisms for protecting sperm. During sperm production and maturation, sperm are inevitably exposed to various oxidative stresses, resulting in the production of excessive amounts of reactive oxygen species such as hydrogen peroxide (H2O2). These reactive oxygen species are like a double-edged sword. In moderate amounts, they participate in physiological processes such as cell signaling, but in excess, they can cause severe damage to sperm. H2O2 is a highly oxidizing agent, attacking biomolecules within sperm, such as DNA, proteins, and lipids. This can lead to DNA fragmentation, protein denaturation, and cell membrane damage, thus impairing normal sperm function.
Spermidine acts like a heroic antioxidant, rapidly reacting with excess H2O2 within sperm, converting it into harmless water and oxygen. This effectively eliminates these reactive oxygen species and mitigates the damage caused by oxidative stress to sperm. Studies have shown that when spermidine in semen exerts its antioxidant properties, H2O2 levels in sperm are significantly reduced, significantly preserving the integrity of sperm DNA. Specifically, sperm DNA fragmentation rates decreased by 22%, meaning more sperm possess intact DNA, enabling better transmission of genetic information and improving fertilization success rates.
Spermidine also plays a key role in regulating sperm motility. Sperm motility is a key factor in its ability to successfully reach the egg and complete fertilization. Male fruit flies deficient in spermidine are like a boat without power; their sperm motility is significantly reduced, resulting in a 50% drop in fertilization rate. This phenomenon clearly demonstrates the importance of spermidine for sperm motility. However, when these male fruit flies were supplemented with spermidine, their sperm motility was significantly restored, and their fertilization rate returned to wild-type levels. This is like refueling a boat without power, allowing it to sail smoothly. Studies on human sperm have also shown that spermidine regulates sperm flagellar motility, providing sufficient power for sperm to swim, enabling them to more quickly and accurately reach the egg and complete fertilization.
(II) Maintenance of Female Reproductive Reserve
In the female reproductive system, the ovary is like a “treasure house of life,” storing numerous follicles, each containing a potential egg. Spermidine, like a loyal guardian of this “treasure trove,” plays a vital role in maintaining the health of ovarian granulosa cells and the normal development of follicles, effectively delaying the depletion of female reproductive reserves and prolonging the reproductive cycle.
In the ovary, granulosa cells surround the egg, providing essential nutrients and support for its development and maturation. However, with aging, increased stress, and various adverse environmental factors, granulosa cells are susceptible to damage and apoptosis. Once granulosa cells undergo apoptosis, the normal development and function of the follicles is compromised, leading to follicle depletion and, in turn, shortening the female reproductive cycle.
Spermidine inhibits apoptosis in ovarian granulosa cells, providing a favorable environment for the development and survival of follicles. Studies have shown that spermidine can regulate the expression of apoptosis-related proteins within cells, increasing the Bcl-2/Bax ratio by 30%. Bcl-2 is an anti-apoptotic protein that inhibits apoptosis; Bax, a pro-apoptotic protein, has the opposite effect of Bcl-2. An elevated Bcl-2/Bax ratio indicates enhanced anti-apoptotic signaling within cells, inhibiting apoptosis. Under the action of spermidine, Bcl-2 expression in ovarian granulosa cells increases, while Bax expression decreases. This effectively inhibits granulosa cell apoptosis, delays follicular exhaustion, and effectively protects against female reproductive failure.
Clinical studies have more clearly demonstrated the close connection between spermidine and female reproductive health. Polycystic ovary syndrome (PCOS) is a common gynecological endocrine disorder, and patients often face ovulatory disorders and infertility. Studies have found that spermidine levels in the follicular fluid of PCOS patients are positively correlated with egg quality (r = 0.62, P < 0.01). This suggests that higher spermidine levels in follicular fluid are associated with better egg quality. Spermidine acts as a barometer of egg quality; its levels directly influence egg development and maturation, and thus, female fertility. By supplementing with spermidine, it is possible to improve the quality of eggs in women with PCOS, increase their chances of fertility, and bring new hope to women who desire to become mothers.
Scientific Supplementation and Research Outlook
Spermidine’s six major benefits, including autophagy, anti-aging, cardiovascular protection, immune regulation, neuroprotection, and reproductive health regulation, reveal its enormous potential for maintaining human health, slowing the aging process, and preventing and treating various diseases. These benefits do not exist in isolation but rather are interconnected and synergistic, forming a complex and intricate biological regulatory network.
The efficacy of spermidine depends on its dose and tissue distribution. The current recommended dietary intake is 0.3-1.5 mg/day. This dosage range is based on extensive scientific research and practical experience and is designed to ensure that spermidine maximizes its health-promoting effects while maintaining safety. Low doses may not achieve the desired health benefits, while high doses may cause adverse reactions, such as gastrointestinal discomfort.
Wheat germ, aged cheese, soybeans, and mushrooms are all good food sources of spermidine. Wheat germ, for example, contains up to 243mg of spermidine per kilogram. Aged cheese also contains significant amounts, reaching 128mg per kilogram. In daily life, we can meet our body’s spermidine needs by adjusting our diet and increasing our intake of these foods. For those who, for specific reasons, cannot get enough spermidine from food, spermidine supplements may be considered. However, before use, it is important to consult a doctor or nutritionist to ensure safety and effectiveness.
Currently, clinical research on spermidine in areas such as metabolic syndrome and sarcopenia is actively underway. Metabolic syndrome is a complex group of metabolic disorders, including obesity, hypertension, hyperglycemia, and dyslipidemia, which pose a serious threat to human health. Sarcopenia, on the other hand, is a condition characterized by a decrease in muscle mass and strength with aging, which can lead to a decline in quality of life in the elderly and increase the risk of falls and fractures. As a “multi-functional anti-aging molecule,” spermidine has shown potential application in the prevention and treatment of these diseases. By activating autophagy, regulating metabolism, and providing anti-inflammatory and antioxidant benefits, spermidine is expected to provide new strategies and approaches for the treatment of metabolic syndrome and sarcopenia.
With continued research, the potential applications of spermidine in more disease prevention and treatment areas will gradually be revealed. We believe that in the near future, spermidine will make even greater contributions to human health, becoming a powerful weapon in maintaining health and delaying aging.



















 Delaying the Aging Process: The Secret to Longevity from Model Organisms to Humans
Delaying the Aging Process: The Secret to Longevity from Model Organisms to Humans


