Melatonin, an indoleamine hormone secreted by the brain’s pineal gland, plays a crucial role in human sleep regulation. Its secretion is regulated by the fluctuations in light levels during the day and night. As night falls, darkness stimulates the pineal gland, significantly increasing melatonin production. Early morning light acts as a brake, inhibiting melatonin synthesis. This cycle forms the body’s precise “biological clock,” a core signal regulating the sleep-wake cycle. Melatonin can be described as the body’s own “sleep start switch.” Whenever its secretion rises at night, it signals the body to sleep. Normally, its concentration peaks between 2 and 3 a.m., when sleepiness is most intense. Melatonin directly influences the stability of the sleep-wake cycle, ensuring regular, sound sleep.
Age-Related Changes in Secretion and Sleep Degeneration
Melatonin secretion is closely related to age, exhibiting distinct patterns of change. Newborns, whose bodily functions are not yet fully developed, produce very low levels of melatonin. As we age and reach puberty, our bodies mature and melatonin secretion reaches its peak, allowing adolescents to generally enjoy relatively sound sleep. However, time is merciless. After age 40, the body’s functional capacity gradually declines, and melatonin secretion levels drop by over 50% compared to youth. Middle-aged and elderly people often complain of difficulty falling asleep and frequent nighttime awakenings, largely related to the physiological decline of melatonin. This age-related change in secretion has become a key reason for people to consider exogenous melatonin supplementation, hoping to alleviate sleep disturbances caused by decreased secretion and restore quality sleep.
 Core Function: Multifaceted Health Benefits Beyond Sleep Aid
Core Function: Multifaceted Health Benefits Beyond Sleep Aid
(I) Precisely Targeted Sleep Regulation
Melatonin plays a precisely targeted role in sleep regulation, primarily by stimulating MT1/MT2 receptors to inhibit the activity of arousal neurons, thereby regulating the sleep-wake cycle. Clinical studies have shown that within a reasonable dose range of 0.5-2mg, melatonin can shorten sleep onset latency by 20%-30%. This means that for those who toss and turn in bed and have difficulty falling asleep quickly, supplementing with an appropriate amount of melatonin can effectively shorten the time it takes to fall asleep, allowing them to more quickly enter a sweet dream.
In addition to shortening the time it takes to fall asleep, melatonin can also optimize sleep structure, increasing the proportion of deep non-rapid eye movement (NREM) sleep, and reducing nighttime awakenings. Deep sleep is crucial for physical recovery and maintaining brain function. An increased proportion of deep sleep allows people to get more restful sleep and wake refreshed. For shift workers, whose working hours are inconsistent with their natural circadian rhythm, their biological clocks are disrupted, leading to frequent sleep disturbances. Melatonin supplementation can help them adjust their sleep rhythms, improve sleep quality, and reduce work errors and fatigue caused by sleep deprivation. For those experiencing jet lag from traveling across time zones, melatonin can also play a vital role, helping them quickly adapt to the new time zone, alleviating the discomfort of jet lag, and restoring a normal sleep and lifestyle.
(II) Antioxidant and Anti-Aging Cellular Protection Mechanisms
Melatonin is a fat-soluble antioxidant with potent antioxidant properties. Its free radical scavenging capacity is twice that of vitamin C, and it can easily penetrate the blood-brain barrier, providing protection for neurons. During normal physiological metabolism, the human body produces free radicals, such as superoxide anion radicals and hydroxyl radicals. These free radicals have strong oxidative activity. If accumulated in large quantities, they attack biomolecules such as lipids, proteins, and DNA within cells, causing oxidative damage, which can lead to aging and various diseases. Melatonin acts like a loyal guardian, promptly scavenging these free radicals, blocking oxidative stress, maintaining a stable intracellular environment, and protecting cells from oxidative damage.
Research has confirmed that melatonin can also slow mitochondrial aging by regulating the expression of longevity genes such as SIRT1. Mitochondria are the “energy factories” of cells, and their functional state is closely related to cellular aging. With aging, mitochondrial function gradually declines, energy production becomes less efficient, and more free radicals are produced, further accelerating the aging process. By regulating SIRT1 gene expression and activating related signaling pathways, melatonin enhances mitochondrial function, reduces free radical production, and slows mitochondrial aging, thereby, to a certain extent, delaying the aging of entire cells and the body. Studies on individuals at high risk for Alzheimer’s disease have shown that melatonin’s antioxidant and cytoprotective effects have potential preventive value. It may reduce the risk of Alzheimer’s disease by reducing oxidative damage to brain neurons, opening up new research directions in this field.
(III) Bidirectional Regulation of Immune and Endocrine Functions
Melatonin plays an important role in immune regulation and can enhance immune surveillance. It can promote T lymphocyte differentiation and enhance natural killer cell activity. T lymphocytes are a crucial component of the immune system, playing a key role in cellular immunity. Their normal differentiation and function are crucial for identifying and eliminating abnormal cells such as pathogens and tumor cells. Natural killer cells can directly kill virus-infected cells and tumor cells, serving as the body’s first line of defense against infection and tumors. For cancer patients, the immune system is severely suppressed after chemotherapy and radiotherapy. Supplementing melatonin during this period can aid immune reconstitution, boost immunity, enhance resistance to pathogens, reduce the incidence of infection, and promote recovery.
Melatonin also plays a crucial role in maintaining endocrine system stability, primarily by inhibiting gonadotropin release and indirectly regulating the reproductive axis. Gonadotropin release is regulated by multiple factors, and melatonin, as one of these regulators, has a significant impact on the development and function of the reproductive system. However, the effects of melatonin on puberty require long-term clinical observation. During puberty, the body’s endocrine system undergoes dramatic changes, reproductive organs gradually mature, and sex hormone levels fluctuate. Using melatonin during this period may have unexpected effects on the endocrine system and interfere with normal puberty development, so caution is advised.
Scientific Dosage: Precise Control of Dosage, Timing, and Method
(I) Individualized Dosage Regimen
Regular Dosage: For most adults, taking melatonin 1-2 hours before bedtime is optimal. Taking 0.5-1mg of melatonin at this time effectively exerts its sleep-inducing properties and helps the body transition to sleep smoothly. This dosage range, validated by extensive clinical research and practical experience, ensures good sleep while minimizing the risk of side effects. However, individual responses to medication vary. Some people may be very sensitive to lower doses, while others may require increased doses to achieve optimal sleep. The maximum daily dose should not exceed 5mg. Excessive use can not only cause symptoms such as drowsiness and dizziness the following day but can also negatively impact the body’s endocrine system and circadian rhythm.
Special Scenarios: Traveling across time zones disrupts the body’s circadian rhythm, leading to jet lag and symptoms such as sleep disturbances, fatigue, and difficulty concentrating. To alleviate these discomforts, people can take 1-2mg of melatonin one hour before their target bedtime for 3-5 consecutive days. This helps the body quickly adjust to the new time zone, resets the body clock, and shortens the period of jet lag, allowing travelers to quickly return to a normal sleep and lifestyle, allowing them to better enjoy their trip or work.
(II) Contraindications and Drug Interactions
Absolute Contraindications: Melatonin should be strictly avoided by pregnant and breastfeeding women. During pregnancy, hormonal levels fluctuate dramatically, and the fetus is undergoing a critical period of development. Using melatonin during this period may pose potential risks to fetal growth and development, interfering with normal fetal physiological functions. If a breastfeeding woman uses melatonin, the drug components may be transferred to the baby through breast milk. Babies, whose bodily functions are not yet fully developed, may have poorer tolerance and metabolism of the drug, potentially resulting in adverse effects on the baby’s health.
Drug Synergy Risk: The dose should be halved when used with benzodiazepine sedatives. Benzodiazepines inherently have strong sedative and hypnotic effects. When used in combination with melatonin, their effects may be additive, leading to excessive central nervous system depression and serious adverse reactions such as respiratory depression and deep coma. Therefore, strict dosage control and caution are essential for combined use.
Drug synergy risk: Anticoagulants such as warfarin primarily exert their anticoagulant effect by inhibiting the activity of coagulation factors. Melatonin may affect platelet function or interfere with the metabolism of anticoagulants, thereby enhancing the anticoagulant effect and increasing the risk of bleeding. If combined use of these two drugs is necessary for treatment, they should be taken at least four hours apart to minimize the risk of drug interactions. During medication use, coagulation parameters, such as the international normalized ratio (INR), should also be closely monitored, and dosage adjustments should be made based on monitoring results to ensure safe use.
Frontier Perspective: Research Breakthroughs from Sleep to Whole-Body Health
(I) New Targets for Chronic Disease Prevention
Melatonin is emerging as a promising new target for chronic disease prevention. Recent clinical studies have revealed promising results, demonstrating its ability to positively impact patients with hypertension by modulating the NF-κB inflammatory pathway. Specifically, melatonin can increase the magnitude of the nocturnal drop in blood pressure by 15%, which is undoubtedly significant news for patients with hypertension. Effective control of nocturnal blood pressure can reduce the risk of cardiovascular and cerebrovascular disease and improve their quality of life.
Coronary heart disease (CHD), a serious threat to human health, is associated with multiple factors. Melatonin significantly reduces the risk of CHD, reducing it by 22%. Research suggests that melatonin may protect the cardiovascular system through various mechanisms, including antioxidant and anti-inflammatory properties and lipid regulation, thereby reducing the risk of CHD. In the area of liver disease, melatonin has shown great potential in repairing liver damage in non-alcoholic fatty liver disease (NAFLD) and is currently entering Phase II clinical trials. NAFLD is a common liver disease, and its incidence is increasing annually due to changes in lifestyles and rising obesity rates. Melatonin has the potential to become a new treatment for NAFLD, bringing hope to patients.
(II) Dosage Form Innovation and Precision Delivery
With the continuous advancement of technology, significant progress has been made in the formulation innovation and precision delivery of melatonin. The emergence of oral fast-dissolving films has effectively addressed the inconvenience of swallowing traditional tablets. This dosage form offers unique advantages: a disintegration time of less than 15 seconds, rapid dissolution in the mouth, and no need for water. This makes it a more convenient option for those with swallowing difficulties, such as the elderly, children, and bedridden patients.
Nasal spray formulations have achieved a significant breakthrough in bioavailability, increasing it to 85%, compared to the 50% bioavailability of traditional tablets. Nasal sprays, administered through the nasal cavity, deliver the drug directly to the nasal mucosa, bypassing the first-pass effect in the liver, thereby improving drug absorption and enabling faster onset of action.
The application of nanoliposome delivery technology offers a new solution for the precise delivery and controlled release of melatonin. This technology enables controlled release over 12 hours, offering a new option for alleviating early morning awakenings. Early morning awakenings are a common problem for many insomniacs. The advent of nanoliposome delivery technology allows for a slow release of melatonin throughout the body, maintaining stable blood concentrations, thereby extending sleep duration and improving sleep quality.
The rational use of melatonin must transcend the single concept of a “sleep aid.” Based on accurate assessment of individual secretion levels, sleep disorder type, and underlying health, standardized dosage and cycle management can be used as a scientific tool for regulating circadian rhythms and preventing and controlling age-related diseases. With further research into its mechanism of action, the potential of melatonin in anti-aging and immune regulation will be further unleashed, ushering sleep health management into the era of precision nutrition.




















 Core Function: Multifaceted Health Benefits Beyond Sleep Aid
Core Function: Multifaceted Health Benefits Beyond Sleep Aid

-672x372.jpg.webp)
.jpg.webp) Core Advantages: Multi-Dimensional Value Beyond Traditional Calcium Supplements
Core Advantages: Multi-Dimensional Value Beyond Traditional Calcium Supplements2.jpg.webp)
1.jpg.webp)

 Core Benefits: Multi-Target Biological Activity Analysis
Core Benefits: Multi-Target Biological Activity Analysis

 Rationally Exploring the Health Value of Plant Polyphenols
Rationally Exploring the Health Value of Plant Polyphenols





















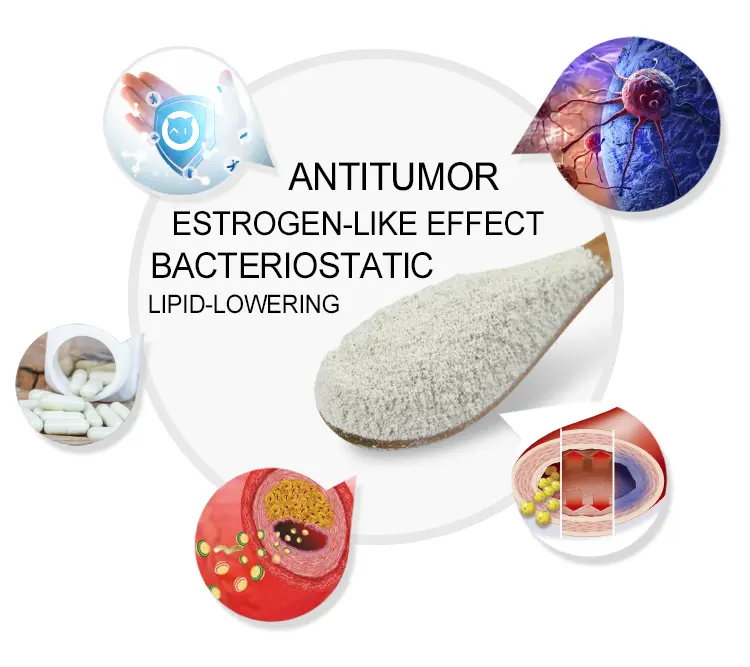
 As a representative natural phytoestrogen, genistein, with its multi-target bioactivity, has opened up new avenues for menopausal management, neuroprotection, and metabolic disease prevention. In menopausal management, it can effectively alleviate symptoms such as hot flashes and night sweats, regulate lipid profiles, and reduce the risk of cardiovascular disease. In terms of neuroprotection, it has shown potential as an intervention for neurodegenerative diseases such as Alzheimer’s disease. In metabolic disease prevention, it plays a positive role through mechanisms such as regulating lipid metabolism. With the continuous innovation of delivery technologies, such as liposome encapsulation and microencapsulation formulations targeting the gut microbiome, and the accumulating clinical evidence, genistein has the potential to evolve from a simple dietary supplement to a precision medicine intervention.
As a representative natural phytoestrogen, genistein, with its multi-target bioactivity, has opened up new avenues for menopausal management, neuroprotection, and metabolic disease prevention. In menopausal management, it can effectively alleviate symptoms such as hot flashes and night sweats, regulate lipid profiles, and reduce the risk of cardiovascular disease. In terms of neuroprotection, it has shown potential as an intervention for neurodegenerative diseases such as Alzheimer’s disease. In metabolic disease prevention, it plays a positive role through mechanisms such as regulating lipid metabolism. With the continuous innovation of delivery technologies, such as liposome encapsulation and microencapsulation formulations targeting the gut microbiome, and the accumulating clinical evidence, genistein has the potential to evolve from a simple dietary supplement to a precision medicine intervention.






 NAD: Structure and Properties
NAD: Structure and Properties

 As a star molecule in the life sciences, NAD has continuously revealed its crucial role in life over the past century of research. From a core player in energy metabolism to a staunch guardian of DNA repair, to its immense potential in aging and disease research, NAD permeates every vital aspect of life.
As a star molecule in the life sciences, NAD has continuously revealed its crucial role in life over the past century of research. From a core player in energy metabolism to a staunch guardian of DNA repair, to its immense potential in aging and disease research, NAD permeates every vital aspect of life.