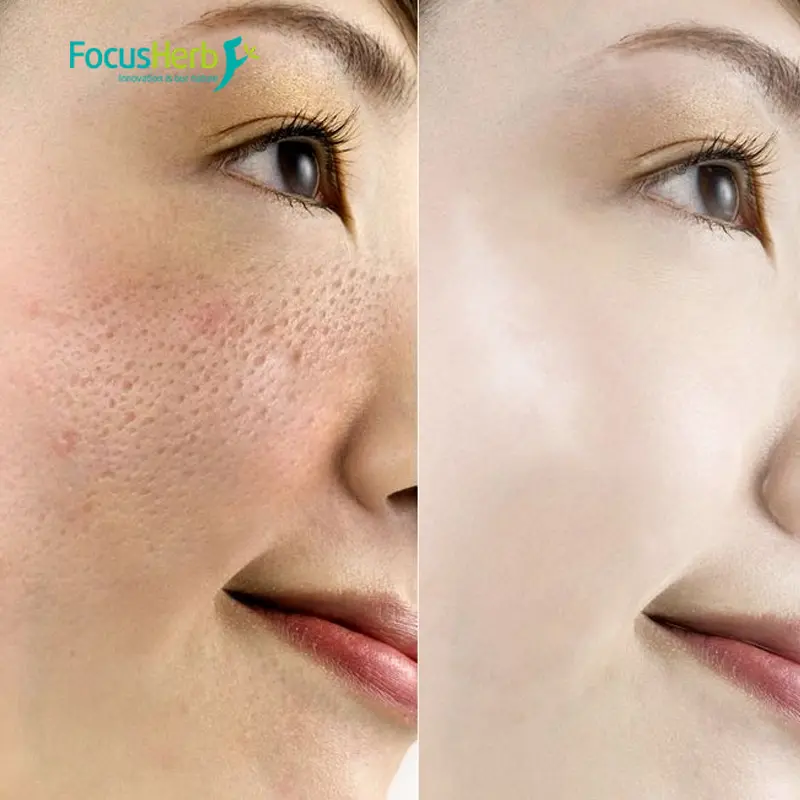
Dihydroquercetin is a hydrogenated derivative of quercetin, a natural flavonoid found in plants such as pine trees, grape seeds, and onions. It possesses antioxidant, anti-inflammatory, and metabolic regulatory properties and is currently being researched for use in food, health supplements, and pharmaceuticals.
Antioxidant defense: the core mechanism of free radical scavenging
In vivo, the production and clearance of free radicals are in a dynamic equilibrium. Once this equilibrium is disrupted, excessive free radicals trigger oxidative stress, damaging cells and tissues, and ultimately leading to a variety of diseases. Dihydroquercetin, as a potent antioxidant, plays a key role in maintaining the body’s redox balance. Its antioxidant mechanism is primarily based on the free radical-snatching ability of the phenolic hydroxyl group and its unique cyclic antioxidant activity.
Free Radical-Snatching Function of the Phenolic Hydroxyl Group
Dihydroquercetin contains five phenolic hydroxyl groups in its molecular structure, which gives it a powerful free radical-snatching ability. From a chemical perspective, free radicals possess unpaired electrons and are extremely reactive. They aggressively steal electrons from other molecules within cells, attacking biomolecules such as DNA, proteins, and lipids, and causing various oxidative damage. The phenolic hydroxyl groups in dihydroquercetin act like “electron donors,” actively donating hydrogen atoms. When free radicals attack, the hydrogen atoms in the phenolic hydroxyl groups bind to the free radicals’ unpaired electrons, stabilizing them and preventing them from further attacking other substances within the cell, thus interrupting the chain reaction initiated by the free radicals.
For example, superoxide anion radicals are a common free radical produced during cellular respiration. If accumulated in large quantities, they attack lipids in the mitochondrial membrane, affecting mitochondrial function and disrupting cellular energy metabolism. Dihydroquercetin reacts rapidly with superoxide anion radicals, donating hydrogen atoms to convert them into relatively stable hydrogen peroxide, thus preventing further damage to mitochondria. Another example is the hydroxyl radical, a highly oxidizing free radical that can directly damage DNA structure and cause serious consequences such as gene mutations. Dihydroquercetin can also bind to hydroxyl radicals through its phenolic hydroxyl groups, effectively reducing the risk of hydroxyl radicals damaging DNA, acting like a protective shield for vital substances within the cell.
Continuous Cycling Antioxidant Activity
Unlike most antioxidants that are consumed once, dihydroquercetin possesses unique cyclic antioxidant properties. When the phenolic hydroxyl group of dihydroquercetin donates hydrogen atoms to combine with free radicals, it forms phenoloxyl radicals. While phenoloxyl radicals are generally unstable and may continue to trigger new oxidative reactions, the phenoloxyl radicals formed by dihydroquercetin have a unique structure and are highly stable. This stability stems from the electron conjugation effect within the molecule, which allows the unpaired electrons of the phenoloxyl radical to be delocalized within the entire molecular structure, thereby reducing the free radical’s activity.
In the intracellular aqueous environment, water molecules can interact with phenoloxyl radicals. The hydrogen atoms in the water molecules bind to the phenoloxyl radicals, allowing the phenoloxyl radicals to regain their hydrogen atoms and return to the original dihydroquercetin molecule. This process is known as “regenerative” antioxidant activity. It’s like a soldier who, despite expending energy (donating hydrogen atoms) in battle, is able to quickly replenish his energy (regaining hydrogen atoms) and continue fighting. For example, when responding to chronic oxidative stress, antioxidants like vitamin C are depleted after scavenging free radicals. However, dihydroquercetin can be continuously regenerated, providing a sustained antioxidant effect. Studies have shown that in models of chronic inflammation, dihydroquercetin’s sustained antioxidant capacity can effectively reduce oxidative damage in inflamed tissues, promote the resolution of inflammation, and provide more lasting protection.
Cardiovascular System Protection: Dual Protection from Blood Vessels to the Myocardium
The cardiovascular system, the body’s “lifeline,” is directly linked to its quality and length of life. Dihydroquercetin, with its unique biological activity, plays a key role in cardiovascular system protection, from maintaining vascular elasticity to safeguarding the health of myocardial cells, providing a multi-dimensional defense for the cardiovascular system.
Maintaining Vascular Elasticity and Improving Microcirculation
In the cardiovascular system, endothelial cells serve as a crucial barrier between blood and the vessel wall. Their normal function is crucial for maintaining vascular health. However, in modern life, unhealthy lifestyles such as a long-term high-salt and high-fat diet, lack of exercise, smoking, and environmental pollution can damage endothelial cells and trigger an inflammatory response. Once the inflammatory response is activated, monocytes adhere to the endothelium, gradually forming atherosclerotic plaques. These thicken and harden the vessel wall, reducing its elasticity and thus impairing normal blood flow. Dihydroquercetin can inhibit inflammatory signaling pathways, such as the nuclear factor-κB (NF-κB) pathway, reducing the release of inflammatory factors such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). This can inhibit the inflammatory response in vascular endothelial cells, prevent monocyte adhesion, and delay the formation of atherosclerotic plaques. A study in an animal model of hyperlipidemia found that dihydroquercetin treatment significantly reduced the expression of inflammatory factors in vascular endothelial cells, significantly reduced monocyte adhesion to the endothelium, and significantly reduced the area of atherosclerotic plaques.
Dihydroquercetin also has the effect of dilating vascular smooth muscle. It acts on vascular smooth muscle cells, activating a series of intracellular signaling pathways, leading to vascular smooth muscle relaxation. Specifically, dihydroquercetin can promote the synthesis and release of nitric oxide (NO) in vascular endothelial cells. NO is a potent vasodilator that rapidly diffuses into vascular smooth muscle cells, activating guanylate cyclase and increasing intracellular cyclic guanosine monophosphate (cGMP) levels, leading to vascular smooth muscle relaxation. This vasodilatory effect effectively reduces vascular resistance, increases coronary blood flow, and ensures adequate blood supply to the heart. Clinical studies have also shown that long-term dihydroquercetin consumption significantly increases endothelial nitric oxide (NO) production, enhances vasodilatory function, and effectively controls blood pressure, offering significant preventive value for cardiovascular diseases such as hypertension and arteriosclerosis.
Myocardial Cell Protection and Energy Metabolism Optimization
Myocardial ischemia-reperfusion injury is a common and serious problem in cardiovascular diseases, particularly in patients with coronary artery disease. When a coronary artery is blocked, myocardial cells are damaged by ischemia. Restoration of blood perfusion triggers a complex series of pathophysiological reactions, leading to further damage and even apoptosis of myocardial cells, severely impairing cardiac function and increasing the risk of sudden cardiac death. Dihydroquercetin demonstrates remarkable protective properties against myocardial ischemia-reperfusion injury. From the perspective of the apoptosis pathway, dihydroquercetin can regulate the expression of apoptosis-related proteins within cells. It upregulates the expression of the anti-apoptotic protein Bcl-2, which acts like a “lifeguard” within the cell, inhibiting apoptosis. Simultaneously, dihydroquercetin downregulates the expression of the pro-apoptotic protein Bax, reducing factors that induce apoptosis, thereby inhibiting the apoptotic pathway in cardiomyocytes and enabling their survival in the harsh environment of ischemia-reperfusion injury.
Mitochondria are the “energy factories” of cells. For cardiomyocytes, which have high energy demands, proper mitochondrial function is particularly crucial. During myocardial ischemia-reperfusion, mitochondria are vulnerable to damage, resulting in a breakdown of their structural integrity, disrupted energy metabolism, and an inability to provide sufficient ATP for cardiomyocytes. Dihydroquercetin can protect mitochondrial structural integrity in multiple ways. Dihydroquercetin has antioxidant properties, scavenging free radicals around mitochondria and reducing oxidative damage to mitochondrial membranes. Furthermore, dihydroquercetin regulates mitochondrial membrane potential, maintaining normal mitochondrial function and ensuring a stable supply of ATP.
A large number of animal studies have provided strong evidence for dihydroquercetin’s cardioprotective effects. In animal models of myocardial infarction, treatment with dihydroquercetin significantly reduced infarct size and improved myocardial contractility. This suggests that dihydroquercetin not only reduces cardiomyocyte death but also promotes functional recovery. This makes it of great significance as an adjunctive therapy for patients with coronary artery disease, offering new strategies and hope for reducing the risk of sudden cardiac death.
Metabolic Regulation: Balancing Blood Glucose and Lipids
In modern life, the incidence of metabolic diseases such as diabetes and hyperlipidemia is increasing, posing a serious threat to human health. Dihydroquercetin, a natural compound with multiple biological activities, demonstrates unique effects in regulating blood glucose and lipids, offering new insights and hope for the prevention and treatment of metabolic diseases.
Improving Insulin Sensitivity and Optimizing Glucose Metabolism
Under normal physiological conditions, insulin is a key hormone that regulates blood glucose levels. It acts like a “key,” unlocking the “door” for cellular glucose uptake, allowing glucose from the blood to enter cells and provide energy for their vital activities. However, in patients with diabetes, especially type 2 diabetes, various factors can reduce cellular sensitivity to insulin, weakening the ability of this “key” to unlock the door. Even when sufficient insulin is secreted, cells are unable to properly uptake glucose, leading to elevated blood glucose and the development of insulin resistance.
Dihydroquercetin has a significant effect in improving insulin resistance and optimizing glucose metabolism. From a cellular signaling perspective, dihydroquercetin can activate the 5′-AMP-activated protein kinase (AMPK) signaling pathway. AMPK is a key regulator of intracellular energy metabolism. When intracellular energy levels decrease, AMPK becomes activated, acting as an intracellular “energy police,” regulating cellular metabolic processes through a series of reactions, increasing energy production and reducing energy expenditure. Dihydroquercetin’s activation of AMPK promotes glucose uptake by skeletal muscle and adipocytes. In skeletal muscle cells, activated AMPK causes glucose transporter 4 (GLUT4) to move from storage vesicles within the cell to the cell membrane surface. GLUT4 acts as a “carrier” for glucose entry into the cell. Increased GLUT4 levels enable more efficient glucose transport from the blood into skeletal muscle cells, thereby lowering blood glucose levels.
In the liver, dihydroquercetin inhibits the activity of key gluconeogenic enzymes, reducing hepatic glucose output and thereby lowering fasting blood glucose levels. Clinical studies have also provided strong evidence for dihydroquercetin’s hypoglycemic effects. In a clinical trial of patients with type 2 diabetes, daily supplementation with 50-100mg of dihydroquercetin resulted in a significant decrease in their glycated hemoglobin levels after a period of intervention. Glycated hemoglobin is an important indicator of average blood sugar levels over the past two to three months. This reduction in levels suggests that dihydroquercetin can effectively control blood sugar levels over the long term, improving the condition of diabetic patients and providing a new natural intervention for diabetes management.
Lipid Metabolism Regulation and Cholesterol Homeostasis
Dyslipidemia refers to abnormally elevated or decreased levels of lipid components in the blood, such as cholesterol, triglycerides, and lipoproteins. It is a significant risk factor for cardiovascular diseases such as atherosclerosis and coronary heart disease. Cholesterol synthesis, transport, and metabolism play key roles in lipid metabolism. Dihydroquercetin can regulate lipid metabolism at multiple levels to maintain cholesterol homeostasis.
Dihydroquercetin can inhibit the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. HMG-CoA reductase is the key rate-limiting enzyme in cholesterol synthesis, acting like the master switch in the cholesterol synthesis production line. When dihydroquercetin inhibits HMG-CoA reductase activity, the cholesterol synthesis production line is partially shut down, reducing cholesterol synthesis in the liver and lowering the source of cholesterol in the blood.
Dihydroquercetin can also promote the expression of low-density lipoprotein (LDL) receptors. LDL receptors act as “LDL receptors” on the cell surface, recognizing and binding to LDL in the blood and transporting it into the cell for metabolic breakdown. When dihydroquercetin promotes LDL receptor expression, the number of “LDL receptors” on the cell surface increases, enabling more efficient uptake of LDL in the blood, accelerating the clearance of “bad cholesterol” (LDL-C) from the blood, and reducing LDL-C levels.
Notably, dihydroquercetin’s antioxidant properties also play a crucial role in regulating lipid metabolism. In the blood, LDL is easily oxidized and modified, forming oxidized LDL (ox-LDL). Ox-LDL is highly cytotoxic and is taken up in large quantities by macrophages, leading to their transformation into foam cells. Foam cells accumulate within the blood vessel walls, gradually forming atherosclerotic plaques. Dihydroquercetin can prevent the oxidative modification of LDL, reduce the formation of ox-LDL, inhibit the initiating factors of atherosclerosis at the source, and protect the cardiovascular system from damage caused by lipid peroxidation products. Numerous animal and cell studies have confirmed these lipid-lowering effects of dihydroquercetin, providing a solid theoretical basis for its use in the prevention and treatment of hyperlipidemia.
Liver Protection: From Cell Regeneration to Fibrosis Intervention
As the largest organ in the human body, the liver undertakes numerous important physiological functions, including metabolism, detoxification, and immune regulation. However, many factors in modern life, such as alcohol consumption, drug abuse, environmental pollution, and poor dietary habits, pose significant challenges to the liver, leading to an increasing incidence of various liver diseases. Dihydroquercetin, with its unique biological activity, has demonstrated significant efficacy in liver protection, from promoting hepatocyte regeneration to inhibiting the progression of liver fibrosis, providing comprehensive protection for liver health.
Regulating the Balance between Hepatocyte Proliferation and Apoptosis
When the liver is damaged, the balance between hepatocyte proliferation and apoptosis is crucial for liver repair and functional recovery. Under normal circumstances, hepatocytes maintain a relatively stable state. However, when the liver is exposed to external factors such as alcohol, drugs, and viral infections, the normal physiological functions of hepatocytes are disrupted, and some hepatocytes undergo apoptosis or necrosis. At this time, the liver needs to activate the hepatocyte proliferation mechanism to replenish the damaged hepatocytes and maintain normal liver structure and function. Dihydroquercetin plays a key role in regulating the balance between hepatocyte proliferation and apoptosis. It activates the cell cycle protein Cyclin D1, pushing hepatocytes from the G1 phase into the S phase, promoting DNA replication and cell division. The cell cycle is a crucial process in cellular life. The G1 phase is the stage of cell growth and preparation for DNA replication, while the S phase is a critical period for DNA replication. Activating Cyclin D1 by dihydroquercetin acts like a supercharged “cell cycle engine” in hepatocytes, accelerating their progression into the DNA replication phase and thereby promoting hepatocyte proliferation.
Concurrently, dihydroquercetin downregulates the pro-apoptotic protein Bax, reducing programmed cell death in hepatocytes. Bax is a pro-apoptotic protein. When activated, it increases mitochondrial membrane permeability, releasing apoptotic factors such as cytochrome C, which in turn activates apoptotic signaling pathways, leading to hepatocyte apoptosis. By downregulating Bax expression, dihydroquercetin acts like a “lock” on hepatocyte apoptosis, reducing apoptosis and creating favorable conditions for hepatocyte regeneration.
Extensive animal studies have provided strong evidence for this effect of dihydroquercetin. In a model of alcoholic liver injury, dihydroquercetin treatment increased the expression of the hepatocyte proliferation marker PCNA by 40%. PCNA is a protein closely associated with DNA synthesis, and its elevated expression indicates enhanced hepatocyte proliferation. This experimental result strongly demonstrates that dihydroquercetin can significantly accelerate liver tissue repair and promote the recovery of damaged livers.
Anti-fibrosis and Inflammatory Microenvironment Modulation
Liver fibrosis is an essential stage in the progression of various chronic liver diseases to cirrhosis. Its primary characteristic is the excessive accumulation of extracellular matrix (ECM) in the liver, leading to structural destruction and functional impairment of liver tissue. Hepatic stellate cell activation plays a central role in the progression of liver fibrosis. When the liver is subjected to sustained injury, hepatic stellate cells become activated, transitioning from a quiescent state to an active state where they proliferate, synthesize, and secrete ECM.
Dihydroquercetin targets hepatic stellate cell activation by inhibiting the transforming growth factor β (TGF-β) signaling pathway. TGF-β is a key cytokine in liver fibrosis, activating hepatic stellate cells and promoting the synthesis and secretion of ECM. By inhibiting the TGF-β signaling pathway, dihydroquercetin acts like a “fuse” for hepatic stellate cell activation, reducing the deposition of ECM components such as type I collagen, thereby halting the fibrotic process.
The liver’s inflammatory microenvironment is also a key factor influencing liver health. Kupffer cells, macrophages within the liver, play a key role in the inflammatory response. When the liver is damaged, Kupffer cells become activated, releasing pro-inflammatory cytokines such as TNF-α and IL-6. These cytokines further exacerbate the liver’s inflammatory response, damaging liver cells. They also stimulate the activation of hepatic stellate cells, accelerating the progression of liver fibrosis.
Dihydroquercetin alleviates liver inflammation by inhibiting the release of pro-inflammatory cytokines such as TNF-α and IL-6 by Kupffer cells. This action, like pouring water on the “flame” of liver inflammation, reduces the damage to liver cells and creates a favorable microenvironment for their repair and regeneration. This dual protective effect of dihydroquercetin is crucial for patients with chronic hepatitis and fatty liver disease, delaying progression to cirrhosis and improving patients’ quality of life and survival rates.
Immunomodulation: Anti-inflammatory, Antimicrobial, and Immune Response Enhancement
Immunomodulation plays a crucial role in the human immune system, acting like a well-trained army, constantly protecting the body from pathogens. Dihydroquercetin, with its unique biological activity, plays a multifaceted role in immunomodulation, from precisely inhibiting inflammatory signaling pathways to exhibiting broad-spectrum antimicrobial activity and enhancing immune cell responses, providing comprehensive support for the human immune system.
Precision Inhibition of Inflammatory Signaling Pathways
Under normal circumstances, the body’s inflammatory response is a self-protective mechanism. When the body is invaded by pathogens or subjected to physical or chemical damage, the immune system rapidly initiates an inflammatory response to eliminate the pathogens and repair damaged tissues. However, when the inflammatory response becomes uncontrolled, the overproduction of pro-inflammatory cytokines can lead to an exaggerated inflammatory response, causing damage to the body and triggering various chronic inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease. Dihydroquercetin plays a key role in regulating inflammatory responses, precisely inhibiting the nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways. NF-κB is a transcription factor ubiquitously present in cells. During inflammatory responses, it acts like a “commander.” Once activated, it translocates from the cytoplasm to the nucleus, initiating the transcription of a series of proinflammatory cytokine genes, leading to the massive release of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). Dihydroquercetin inhibits NF-κB activation, preventing its translocation from the cytoplasm to the nucleus. This deactivates the “signal source” for proinflammatory cytokine release and reduces the production of inflammatory mediators.
The MAPK pathway is also a key pathway for inflammatory signaling, encompassing multiple branches such as the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK. When cells are stimulated by inflammation, the MAPK pathway is activated. Through a series of phosphorylation cascades, inflammatory signals are transmitted to the cell nucleus, promoting the expression of inflammation-related genes. Dihydroquercetin can inhibit the activity of key kinases in the MAPK pathway, blocking the transmission of inflammatory signals and reducing the synthesis and release of proinflammatory cytokines.
Clinical studies have provided strong evidence for the anti-inflammatory effects of dihydroquercetin. In a clinical trial of patients with rheumatoid arthritis, daily dihydroquercetin supplementation significantly reduced joint swelling and pain, and significantly decreased blood levels of proinflammatory cytokines such as IL-6 and TNF-α. This suggests that dihydroquercetin’s anti-inflammatory effects are comparable to those of nonsteroidal anti-inflammatory drugs (NSAIDs), but without the common gastrointestinal side effects of NSAIDs, offering a safer and more effective natural option for the treatment of chronic inflammatory diseases.
Broad-Spectrum Antimicrobial Effects and Immune Cell Activation
In our daily lives, we are constantly threatened by various pathogens, including bacteria, viruses, and fungi. Dihydroquercetin exhibits broad-spectrum antimicrobial activity, providing an important line of defense for our health. In vitro studies have demonstrated significant inhibitory effects against a variety of common pathogens, including Staphylococcus aureus, Escherichia coli, and Candida albicans.
In terms of its mechanism of action, dihydroquercetin exerts its antimicrobial activity primarily by disrupting the integrity of pathogen cell membranes and interfering with DNA synthesis. For Staphylococcus aureus, dihydroquercetin interacts with the phospholipid molecules on the cell membrane, disrupting its structure and increasing its permeability, leading to the outflow of intracellular substances and ultimately causing bacterial death. In studies on Escherichia coli, dihydroquercetin was found to inhibit the activity of DNA gyrase, an essential enzyme in bacterial DNA replication. Inhibiting its activity prevents bacterial DNA replication from proceeding normally, thereby inhibiting bacterial growth and reproduction.
In addition to its direct antibacterial effects, dihydroquercetin can also enhance the body’s immune response by promoting T lymphocyte proliferation and natural killer (NK) cell activity, thereby improving the body’s ability to eliminate pathogens. T lymphocytes are the core cells of cellular immunity. Upon recognizing pathogens, they become activated, rapidly proliferating and differentiating into effector T cells. These effector T cells can directly attack and eliminate pathogen-infected cells. Dihydroquercetin can provide a favorable environment for T lymphocyte proliferation, promoting their division and differentiation, and enhancing cellular immunity.
NK cells are a key component of the body’s innate immunity, capable of directly killing virus-infected and tumor cells without prior antigen exposure. Dihydroquercetin can activate NK cells, enhancing their activity and releasing more cytotoxic substances, such as perforin and granzymes. These substances can puncture the cell membranes of infected cells, leading to cell death and effectively clearing virus-infected cells from the body. This immunomodulatory effect of dihydroquercetin has potential therapeutic value in viral diseases such as influenza and herpes, helping patients boost their immunity and better fight viral infections.
Trace element synergy: A golden partnership for enhanced performance
In the complex and delicate human body, various nutrients do not exist in isolation; rather, they collaborate and influence each other to maintain normal physiological functions. Dihydroquercetin and various trace elements exhibit a close synergistic relationship. This synergistic effect not only enhances dihydroquercetin’s biological activity but also promotes its absorption and utilization, bringing comprehensive benefits to human health.
Synergistic Antioxidant Network of Magnesium, Zinc, and Iron
In the human body’s antioxidant defense system, trace elements such as magnesium, zinc, and iron form a highly effective synergistic antioxidant network with dihydroquercetin. Magnesium ions, as a crucial cofactor for superoxide dismutase (SOD), play a key role in the antioxidant process. SOD is a core member of the human antioxidant enzyme system, catalyzing the conversion of superoxide anion radicals into hydrogen peroxide, thereby effectively scavenging superoxide anion radicals from the body and reducing their damage to cells. When dihydroquercetin works synergistically with magnesium ions, it significantly activates SOD activity, injecting a powerful boost into this “antioxidant machine,” enabling it to more efficiently scavenge superoxide anion free radicals. Studies have shown that the combined action of dihydroquercetin and magnesium ions can increase SOD activity by 30%-50%, significantly enhancing the body’s ability to scavenge superoxide anion free radicals and reducing cellular damage caused by oxidative stress.
Zinc ions also play a crucial role in this synergistic antioxidant network. They enhance dihydroquercetin’s ability to bind to cell membranes, allowing it to more easily penetrate the cell interior and expand its free radical scavenging range. The cell membrane is a crucial barrier and a primary target of free radical attack. When dihydroquercetin binds tightly to the cell membrane, it forms an “antioxidant defense line” on the cell membrane surface, promptly scavenging invading free radicals and protecting the integrity and function of the cell membrane. Zinc ions also regulate the composition and activity of many enzymes, including some involved in antioxidant activity, such as glutathione peroxidase (GSH-Px), further enhancing the body’s antioxidant capacity.
Iron ions play a vital role in human physiological processes. However, free iron ions can generate hydroxyl radicals through the Fenton reaction. Hydroxyl radicals are highly oxidizing free radicals that are extremely destructive to cells. Dihydroquercetin has the ability to chelate free iron, forming a stable complex with it. This reduces its participation in the Fenton reaction and reduces the production of hydroxyl radicals. In this process, dihydroquercetin acts as an “iron steward,” properly “safekeeping” free iron ions and preventing them from triggering harmful oxidative reactions, thus protecting cells from hydroxyl radical damage.
This multi-element collaborative “antioxidant matrix” significantly enhances the antioxidant efficacy of individual ingredients. Studies have found that when dihydroquercetin works synergistically with trace elements such as magnesium, zinc, and iron, its antioxidant capacity is 3-5 times greater than when dihydroquercetin is used alone. This synergistic antioxidant network can more effectively protect human cells from external oxidative stressors such as environmental pollution and ultraviolet radiation, reducing the risk of oxidative damage and preventing the onset of various chronic diseases.
Optimizing Nutrient Absorption and Bioavailability
During the human nutrient absorption process, trace elements and dihydroquercetin interact closely, significantly optimizing nutrient absorption and bioavailability and providing more adequate nutritional support.
Trace elements play a crucial role in promoting dihydroquercetin’s absorption across the gastrointestinal mucosa. Dihydroquercetin is both fat-soluble and water-soluble, requiring specialized transport mechanisms for its absorption. Trace elements such as zinc, iron, and magnesium can bind to dihydroquercetin, forming complexes. These complexes can utilize the trace element’s own transporters to more smoothly cross the gastrointestinal mucosa and enter the bloodstream. For example, zinc ions can form stable complexes with dihydroquercetin, leveraging zinc transporters on the surface of intestinal epithelial cells to carry dihydroquercetin into the cells, thereby promoting its absorption. Studies have shown that the synergistic effect of trace elements can increase the bioavailability of dihydroquercetin by over 60%, significantly enhancing its efficacy in the body.
Dihydroquercetin also promotes the absorption of trace elements. It modulates the intestinal microenvironment, affecting the solubility and presence of trace elements, making them more easily absorbed. Within the intestine, dihydroquercetin binds to iron ions, forming a more readily absorbed complex, increasing iron solubility and promoting its absorption. For populations at risk of trace element deficiencies, such as vegetarians, pregnant women, and the elderly, the synergistic effect of dihydroquercetin and trace elements can better meet their trace element needs and prevent the development of diseases associated with trace element deficiencies.
In terms of nutritional support for the nervous system, zinc ions are particularly important in assisting dihydroquercetin in crossing the blood-brain barrier. The blood-brain barrier is a crucial barrier protecting the central nervous system, preventing many harmful substances from entering the brain while also restricting the entry of some nutrients. Zinc ions bind to dihydroquercetin, altering its molecular structure and physicochemical properties, making it easier to cross the blood-brain barrier. Once in the brain, dihydroquercetin exerts its antioxidant and anti-inflammatory effects, scavenging free radicals in the brain, inhibiting neuroinflammatory responses, and enhancing antioxidant protection of the central nervous system. This provides new avenues and hope for the prevention of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Supplementing with dihydroquercetin and trace elements such as zinc may help maintain brain health and slow the onset and progression of neurodegenerative diseases.

Conclusion: Scientific Applications and Future Prospects of Dihydroquercetin
Dihydroquercetin, a natural compound with a wide range of biological activities, demonstrates remarkable efficacy in antioxidant, metabolic, organ, and immune regulation through its unique molecular structure and multi-target mechanisms, providing comprehensive support for human health. From free radical scavenging at the molecular level to regulating signaling pathways at the cellular level, and ultimately to maintaining overall organ function, dihydroquercetin’s effects permeate multiple aspects of human physiology, and its importance is self-evident.
In 2021, the National Health Commission of China approved dihydroquercetin (purity ≥95%) as a new food ingredient. This significant move opens new avenues for the widespread application of dihydroquercetin in the food and health sectors. In the functional food sector, dihydroquercetin, with its outstanding antioxidant and health-promoting properties, is expected to become a core ingredient in a new generation of functional foods, providing consumers with healthier and more functional food options. For example, adding dihydroquercetin to beverages, fermented milk, cocoa products, and other foods not only enhances their nutritional value but also imparts unique health properties, satisfying consumer demand for healthy foods.
Dihydroquercetin also holds promising application prospects in the pharmaceutical and healthcare sectors. With increasing public awareness of health, the demand for natural, safe, and effective health products is also growing. As a natural bioactive substance, dihydroquercetin has low toxicity and side effects, meeting modern consumers’ pursuit of health products. In the future, it may be developed into various health supplements, such as capsules, tablets, and oral solutions, to provide strong support for daily health maintenance. Furthermore, in drug development, dihydroquercetin’s multi-target mechanism of action makes it a potential lead compound for treating a variety of diseases, providing new insights and directions for new drug development. Looking ahead, dihydroquercetin research still has many promising avenues for further exploration. In the field of cancer prevention, although studies have shown that dihydroquercetin has inhibitory effects on various cancer cells, further research is needed to understand its specific mechanisms of action and clarify its targets during tumor development, progression, and metastasis, thereby providing a more solid theoretical foundation for cancer prevention and treatment. In the field of neuroprotection, with the aging population, the incidence of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease is increasing year by year, placing a heavy burden on society and families. Dihydroquercetin has shown promising potential in protecting the nervous system. Future research could focus on its protective mechanisms for nerve cells, its regulation of neurotransmitters, and its inhibitory effects on neuroinflammation, exploring its application in the prevention and treatment of neurodegenerative diseases.
With the continuous advancement of science and technology, precision medicine has become a key trend in medical development. As a natural active ingredient, dihydroquercetin has the potential to be deeply integrated with precision medicine. By analyzing multi-omics data, including individual genetics and proteomics, we can precisely determine the effects and optimal dosage of dihydroquercetin for different individuals, enabling personalized health management and disease treatment. This not only enhances the efficacy of dihydroquercetin but also maximizes its health-promoting effects, providing safer and more effective solutions for human health.
Dihydroquercetin, as a highly promising natural compound, has demonstrated significant value in multiple fields. We believe that with continued in-depth research and expanded applications, dihydroquercetin will play an even more important role in human health, bringing greater benefits to people’s health.




































 Adaptive Applications for Specific Physiological Scenarios
Adaptive Applications for Specific Physiological Scenarios






 The Benefits of Phycocyanin Revealed
The Benefits of Phycocyanin Revealed



 Coenzyme Q10 plays multiple key roles in the human body and is crucial for maintaining good health.
Coenzyme Q10 plays multiple key roles in the human body and is crucial for maintaining good health.